wire plant trellis
Latest articles
wire plant trellis
...
wire plant trellis 【wire plant trellis】
Read Morewire plant trellis
...
wire plant trellis 【wire plant trellis】
Read Morewire plant trellis
...
wire plant trellis 【wire plant trellis】
Read Morewire plant trellis
...
wire plant trellis 【wire plant trellis】
Read More
wire plant trellisHexagonal net structure can be used for slope support, excavation, rock face net mine support form, slope vegetation (green color), railway road isolation block, it can be made into a cage, pad, used for river, flood dam and seawall erosion protection and reservoir, river closed cage. Driving and guiding rivers and waterlogging disasters are major water erosion damage to rivers, resulting in flooding, resulting in significant loss of property and loss of water and soil. Because of this, in dealing with these problems, the application of the living grid structure becomes a solution plan, which makes it possible to protect the shore forever.
...
wire plant trellis 【wire plant trellis】
Read More
wire plant trellisWire mesh first welding after galvanized is through the end of welding and then cold this company to produce wire mesh wire mesh steel mesh construction mesh floor heating mesh galvanized or hot dip galvanized. First galvanized after welding is to change wire mesh, after the end of welding can see the solder joint; The two processes are different in price. The cost of plating after welding is low, the appearance is smooth, and the cost of plating after welding is high, and it is not easy to rust.
...
wire plant trellis 【wire plant trellis】
Read MoreD, coating adhesion; The zinc layer of the plating parts should be firmly combined with the base metal with sufficient adhesion strength, and will not fall off or bulge after the hammer test.
wire plant trellis...
wire plant trellis 【wire plant trellis】
Read More
wire plant trellisWhen the barbed rope is installed in different places, the choice of barbed rope column is different. Usually, the barbed rope column we install is cement column, but the use of cement column also has its drawbacks. For example, it’s not so convenient if we install it on a steep hill where transportation is not very convenient. This time can use barbed rope with the composite column to install.
...
wire plant trellis 【wire plant trellis】
Read More
wire plant trellisLarge rolls of galvanized wire in oxygen violently extinguished, spark, and sodium block, magnesium strip in oxygen extinguished, no spark phenomenon. This phenomenon is determined by the composition of galvanized wire. The galvanized wire used in the experiment is pig iron or steel wire, pig iron and steel are iron and carbon alloy, containing iron and carbon two elements of complex material. Carbon in galvanized wire will produce carbon dioxide when extinguished, solid into gas, volume will shrink rapidly.
...
wire plant trellis 【wire plant trellis】
Read MoreFirst of all, the protective layer of zinc-iron alloy can prevent the steel wire from being corroded by oxidation. The zinc metal reacts with water and oxygen to form a dense zinc oxide layer, which forms a physical barrier to prevent oxygen and water from entering the inside of the steel wire, reducing the chance of corrosion. Compared with ordinary steel wire, hot-dip galvanized wire is more resistant to corrosion in humid, high humidity or corrosive environments such as acid and alkali, so it is more durable.
wire plant trellis...
wire plant trellis 【wire plant trellis】
Read More
Popular articles
Crevice corrosion is a kind of corrosion in small area, especially in concealed position, which can form vicious corrosion cycle. Almost all the crevice corrosion can occur in a metal alloy, with gas containing active anionic neutral medium Z is easy to cause crevice corrosion, crevice corrosion often occurs in the aperture of 0.025 to 0.1 mm, because of long time gathered, the cracks will exist a series of impurities, coupled with the external environment of damp easily corrode the area of the gap is small.
- Hook net use value is still very high, has a very good sex, and its manufacturing concise generous, beautiful, not in high temperature will fade, you can save a sum of maintenance costs, but also according to the different needs of the site to change the shape, to meet the needs of the site.
- 3, look at the packaging, the normal packaging appearance is to use a layer of kraft paper paper with film to package our welded welding net, so that it will not be oxidized and rusted in transit.
The barbed wire factory will store the blade barbed wire inventory in the appropriate place, because it understands the product characteristics.
Latest articles
-
-
Post time: 03-02-23 -
Post time: 04-04-23 -
Post time: 14-07-22 -
-
Heavy hexagonal mesh is made of low carbon steel wire galvanized large wire braided, the tensile strength of steel wire is not less than 38kg/m2, the diameter of steel wire can reach 2.0mm-3.2mm, the surface of steel wire is usually hot galvanized protection, galvanized amount can reach 500g/m2.
Links
As a widely used substance with multiple applications, research is being carried out to improve the production process to reduce the levels of chemicals used and waste produced, and to recycle any by-products.
- * Customer Service The supplier should provide excellent customer service, including prompt delivery, responsive technical support, and easy return policies.
- Titanium Dioxide A Versatile Wholesale Ingredient
- 3. Chemical Stability TiO2 is chemically stable and does not react with other ingredients in cosmetic formulations. This makes it a reliable and long-lasting ingredient in various cosmetic products.
The FDA's Code of Federal Regulations allows for the legal, regulated use of titanium dioxide in food products, under some restrictions.
3. Solubility: insoluble in water.
A 2012 study published in the journal Environmental Science & Technology noted that children are especially exposed to titanium dioxide because of the food that contains the food additive and is particularly marketed to children, including candy and cakes.

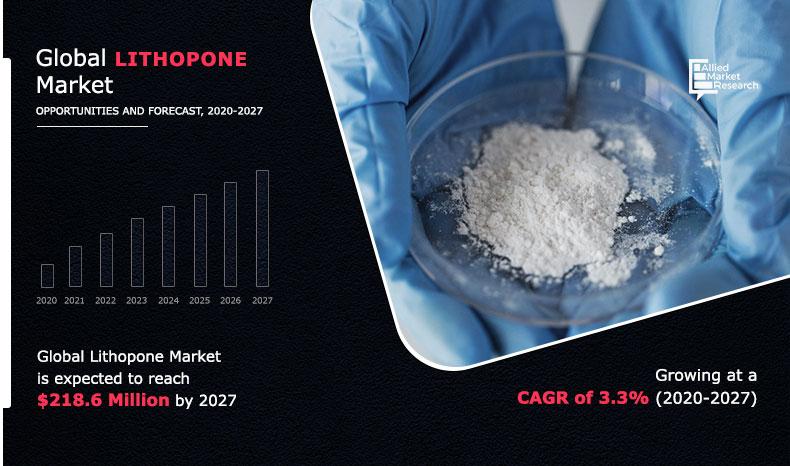
2. Barite calcination method A solution of barium sulfide is prepared. The sulfuric acid and zinc oxide are reacted, purified by adding potassium permanganate and zinc powder, and compressed to obtain a zinc sulfate solution. Then, the prepared barium sulfide solution is mixed and subjected to a metathesis reaction to obtain a mixture of zinc sulfide and barium sulfate, and then the precipitate is subjected to pressure filtration, calcination, wet grinding, drying, and pulverization to obtain a zinc white product.
Lithopone or sulphide of zinc white has been in general use for twenty years or more in many industries where a white pigment of considerable body or hiding power is required that is not subject to change like lead carbonate and has not the brittle character of zinc oxide, besides being sold at a lower figure than either of these. Nevertheless it is still comparatively new to the general painting trade. Because of our tariff protection its manufacture in this country has made great progress. Yet in spite of this and the duty imposed on it, the imports are still in excess of the quantity manufactured here. A short history of its origin will no doubt prove of interest to our readers.
Cet article traite de la découverte de lithopone phosphorescent sur des dessins à l'aquarelle, datés entre 1890 et 1905, de l'artiste Américain John La Farge et de l'histoire du lithopone dans l'industrie des pigments à la fin du 19e et au début du 20e siècle. Malgré de nombreuses qualités souhaitables pour une utilisation en tant que blanc dans les aquarelles et les peintures à l'huile, le développement du lithopone comme pigment pour artistes a été compliqué de par sa tendance à noircir lorsqu'il est exposé au soleil. Sa disponibilité et son usage par les artistes demeurent incertains parce que les catalogues des marchands de couleurs n'étaient généralement pas explicites à indiquer si les pigments blancs contenaient du lithopone. De plus, lors d'un examen visuel, le lithopone peut être confondu avec le blanc de plomb et sa phosphorescence de courte durée peut facilement être ignorée par l'observateur non averti. À ce jour, le lithopone phosphorescent a seulement été documenté sur une autre œuvre: une aquarelle de Van Gogh. En plus de l'histoire de la fabrication du lithopone, cet article décrit le mécanisme de sa phosphorescence et son identification à l'aide de la spectroscopie Raman et de la spectrofluorimétrie. En este artículo se discute el descubrimiento del litopón fosforescente en dibujos a la acuarela por el artista americano John La Farge, fechados de 1890 a 1905, y la historia del litopón en la industria de los pigmentos a finales del Siglo XIX y principios del Siglo XX. A pesar de tener muchas cualidades deseables para su uso en pintura para acuarela o pinturas al óleo blancas, el desarrollo del litopón como pigmento para artistas fue obstaculizado por su tendencia a oscurecerse con la luz solar. Su disponibilidad para los artistas y su adopción por ellos sigue siendo poco clara, ya que por lo general los catálogos comerciales de los coloristas no eran explícitos al describir si los pigmentos blancos contenían litopón. Además, el litopón se puede confundir con blanco de plomo durante el examen visual, y su fosforescencia de corta duración puede ser fácilmente pasada por alto por el observador desinformado. A la fecha, el litopón fosforescente ha sido documentado solamente en otra obra mas: una acuarela por Van Gogh. Además de la historia de la fabricación del litopón, el artículo detalla el mecanismo para su fosforescencia, y su identificación con la ayuda de espectroscopía de Raman, y de espectrofluorimetría. Este artigo discute a descoberta de litopônio fosforescente em desenhos de aquarela do artista americano John La Farge datados de entre 1890 e 1905 e a história do litopônio na indústria de pigmento no final do século XIX e início do século XX. Apesar de ter muitas qualidades desejáveis para o uso em aquarela branca ou tintas a óleo, o desenvolvimento do litopônio como um pigmento de artistas foi prejudicado por sua tendência a se escurecer na luz solar. Sua disponibilidade para e uso por parte de artistas ainda não está clara, uma vez que os catálogos comerciais dos vendedores de tintas geralmente não eram explícitos na descrição de pigmentos brancos como algo que contém litopônio. Além disso, o litopônio pode ser confundido com o branco de chumbo durante o exame visual e sua fosforescência de curta duração pode ser facilmente perdida pelo observador desinformado. O litopônio fosforescente foi documentado em apenas um outro trabalho até hoje: uma aquarela de Van Gogh. Além da história da manufatura do litopônio, o artigo detalha o mecanismo para a sua fosforescência e sua identificação auxiliada pela espectroscopia de Raman e espectrofluorimetria.
Lithopone, white powder, relative density: 4.136 ~ 4.39 g / mL, insoluble in water. It is a mixture of zinc sulfide and barium sulfate. Inorganic white pigment, widely used in plastics such as polyolefin, vinyl resin, ABS resin, polystyrene, polycarbonate, nylon and polyoxymethylene, and white pigments of paints and inks. It is less effective in polyurethane and amino resins and less suitable in fluoroplastics. It is also used for coloring of rubber products, paper, varnish, tarpaulin, leather, watercolor paint, paper, enamel, and the like. Used as a binder in the production of electric beads.
Research has shown that, when ingested as a food additive, titanium dioxide and its nanoparticles can impact, alter, and/or damage important protective bacteria in the gut, along with the metabolic pathways of gut bacteria.
Metal detectors can not only detect a variety of metals at various depths depending on the size of the object, but some can even detect the differences between various metals. This differentiation is done by measuring the deflection of the magnetic field generated by the metal detector. Titanium is often used in medical implants, so patients with implants that contain titanium often have to make this known to airport security personnel in order to pass inspection.
 Some of the most well-known manufacturers include DuPont, Cristal Global, and Huntsman Corporation Some of the most well-known manufacturers include DuPont, Cristal Global, and Huntsman Corporation
Some of the most well-known manufacturers include DuPont, Cristal Global, and Huntsman Corporation Some of the most well-known manufacturers include DuPont, Cristal Global, and Huntsman Corporation honey bun ingredients titanium dioxide manufacturers. These companies have a long history of producing high-quality titanium dioxide products that are safe for human consumption.
honey bun ingredients titanium dioxide manufacturers. These companies have a long history of producing high-quality titanium dioxide products that are safe for human consumption.What's the Verdict?


